Learn The Secrets Of Flapper Chic: A Step-By-Step Guide To Master &Quot;20S-Style Hair&Quot;
Delve into the Realm of Lewis Dot Structures: A Comprehensive Tutorial
A Lewis dot structure tutorial unveils the art of depicting valence electrons, the outermost electrons responsible for chemical bonding, using dots. Like a molecular map, it aids in visualizing electron arrangements and understanding chemical behavior. Take water, for instance: its Lewis dot structure reveals the sharing of electrons between hydrogen and oxygen atoms, explaining its polarity and reactivity.
Lewis dot structures hold immense importance in chemistry. They provide a visual representation of molecules, enabling chemists to predict their properties, bonding patterns, and reactivity. This knowledge is crucial for designing new materials, unraveling chemical reactions, and comprehending the intricate world of molecular interactions. Historically, the development of Lewis dot structures can be traced back to the pioneering work of Gilbert N. Lewis in 1916, who introduced the concept of electron pairs and laid the foundation for understanding chemical bonding.
Delving deeper into this comprehensive tutorial, we will embark on a journey to master the art of drawing Lewis dot structures. We will explore step-by-step guidelines, delve into exceptions and special cases, and uncover the intricacies of resonance structures. Along the way, we will uncover the hidden stories behind molecules, unraveling their electronic configurations and unlocking the secrets of their chemical behavior.
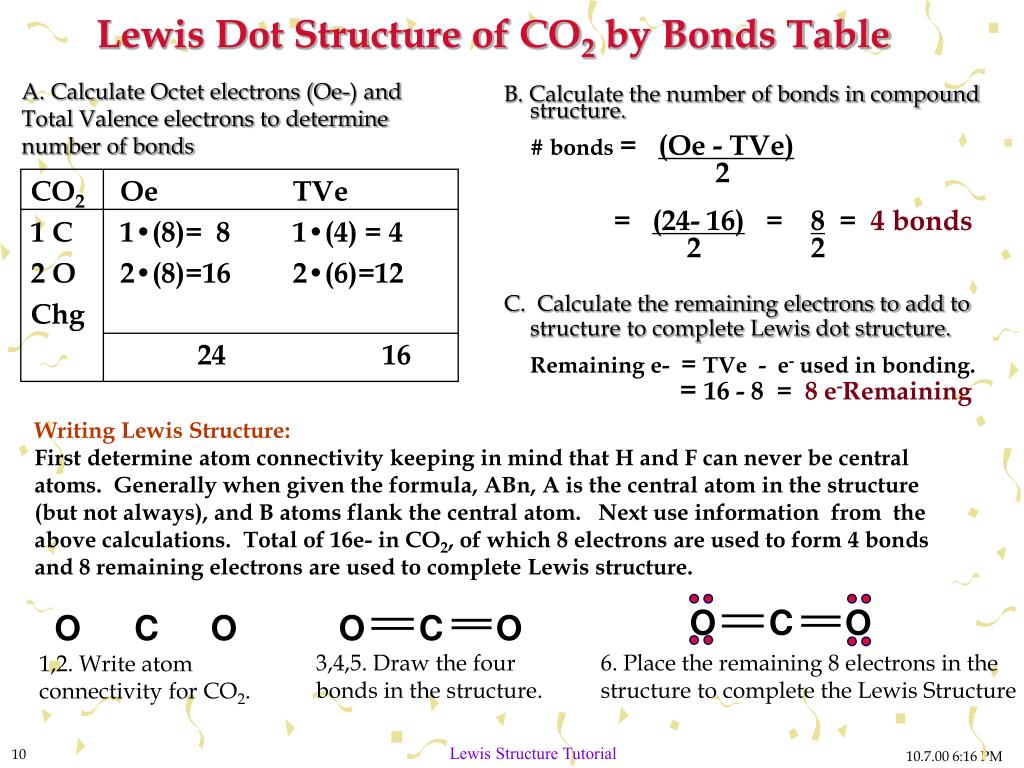
Lewis Dot Structure Tutorial
Lewis dot structure tutorials introduce a foundational concept in chemistry, providing a visual representation of molecules' electronic configurations and chemical bonding patterns. These tutorials hold immense value for comprehending molecular behavior and predicting chemical reactions. Here are ten essential aspects of Lewis dot structure tutorials:
- Definition: Depicting valence electrons using dots.
- Representation: Shows electron arrangements and bonding.
- Prediction: Foretells chemical properties and reactivity.
- Valence Electrons: Electrons involved in bonding.
- Electron Pairs: Shared or unshared electrons.
- Octet Rule: Atoms aim for eight valence electrons.
- Exceptions: Some atoms deviate from the octet rule.
- Resonance: Multiple valid Lewis structures for a molecule.
- Formal Charge: Assigning charges to atoms in a Lewis structure.
- Challenges: Complex molecules require advanced techniques.
Examples illustrate these points. Water's Lewis dot structure reveals two shared electron pairs between oxygen and hydrogen, explaining its polarity. Resonance in benzene involves alternating double and single bonds, contributing to its stability. Formal charges help determine the most stable Lewis structure for complex molecules. By understanding these key points, learners gain a deeper comprehension of chemical bonding and molecular behavior, laying the groundwork for further exploration in chemistry.
Definition
Within the realm of Lewis dot structure tutorials, the fundamental concept of depicting valence electrons using dots takes center stage. Valence electrons, the outermost electrons of an atom, play a pivotal role in chemical bonding and determining an atom's reactivity. By representing these valence electrons as dots placed around the atomic symbol, Lewis dot structures provide a visual representation of an atom's electronic configuration and bonding potential.
- Valence Electrons: The dots in a Lewis dot structure represent the valence electrons of the atom, which are the electrons that participate in chemical bonding.
- Electron Pairs: Valence electrons can exist as lone pairs (two dots together) or as shared pairs (one dot from each atom) in a covalent bond.
- Octet Rule: Many atoms follow the octet rule, which states that atoms tend to gain, lose, or share electrons until they have eight valence electrons.
- Exceptions: Some atoms, such as hydrogen and helium, do not follow the octet rule and have stable Lewis dot structures with fewer or more than eight valence electrons.
Representation
Within the realm of Lewis dot structure tutorials, the representation of electron arrangements and bonding plays a pivotal role in comprehending molecular structure and chemical behavior. By visually depicting the distribution of valence electrons, Lewis dot structures provide a powerful tool for understanding how atoms interact and form molecules.
Cause and Effect:The representation of electron arrangements and bonding in Lewis dot structure tutorials directly influences the ability to predict and explain chemical properties and reactions. By visualizing the electronic configuration of molecules, chemists can gain insights into their stability, reactivity, and bonding patterns.
Components:The representation of electron arrangements and bonding is an essential component of Lewis dot structure tutorials, serving as the foundation for understanding chemical bonding and molecular interactions. It enables chemists to identify lone pairs, shared pairs, and formal charges, which are crucial for predicting molecular properties and reactivity.
Examples:The application of Lewis dot structures in predicting molecular geometry is a compelling example of the significance of representing electron arrangements and bonding. By visualizing the distribution of valence electrons, chemists can determine the molecular shape and bond angles, providing insights into physical and chemical properties.
Applications:The practical significance of understanding the representation of electron arrangements and bonding in Lewis dot structure tutorials extends to various fields, including drug design, materials science, and catalysis. By accurately depicting electron configurations, chemists can design molecules with specific properties, optimize chemical reactions, and develop new materials with tailored functionalities.
Summary:In conclusion, the representation of electron arrangements and bonding in Lewis dot structure tutorials is a fundamental aspect that enables chemists to visualize and understand molecular structure and chemical behavior. This representation serves as a cornerstone for predicting properties, explaining reactions, and designing new materials, contributing to advancements across various scientific disciplines.
Prediction
Within the realm of Lewis dot structure tutorials, the ability to predict chemical properties and reactivity stands as a pivotal aspect that elevates its significance in the field of chemistry. This predictive power stems from the inherent relationship between electron arrangements and molecular behavior.
Cause and Effect:The predictive nature of Lewis dot structure tutorials directly influences the understanding of chemical properties and reactivity. By visualizing the distribution of valence electrons, chemists can infer the stability, polarity, and bonding patterns of molecules. These insights, in turn, enable the prediction of chemical reactivity, reaction pathways, and the design of new materials with tailored properties.
Components:Prediction is an integral component of Lewis dot structure tutorials, as it provides a framework for interpreting and understanding the behavior of molecules. It complements the representation of electron arrangements and bonding by offering a dynamic perspective that connects electronic structure to chemical properties and reactivity.
Examples:A compelling example lies in the prediction of molecular polarity. By examining the Lewis dot structure of a molecule, chemists can determine the distribution of partial charges and identify polar bonds. This information is crucial for understanding intermolecular interactions, such as hydrogen bonding and dipole-dipole interactions, which influence physical properties like solubility and boiling point.
Applications:The practical significance of predictive Lewis dot structure tutorials extends to various fields. In drug design, Lewis dot structures aid in predicting drug-receptor interactions and optimizing drug efficacy. In materials science, they facilitate the design of materials with specific electronic and optical properties. Furthermore, in catalysis, Lewis dot structures help elucidate reaction mechanisms and design catalysts with enhanced activity and selectivity.
Conclusion:In conclusion, the predictive power of Lewis dot structure tutorials empowers chemists with the ability to unravel the intricacies of chemical properties and reactivity. This predictive capability finds applications in diverse fields, driving advancements in drug design, materials science, catalysis, and beyond. Despite the challenges in accurately predicting complex chemical behavior, Lewis dot structure tutorials remain an indispensable tool for chemists seeking to understand and manipulate the molecular world.
Valence Electrons
Within the context of Lewis dot structure tutorials, the concept of valence electrons, the electrons involved in bonding, takes center stage. These electrons play a pivotal role in determining the chemical properties and bonding behavior of atoms and molecules.
- Definition: Valence electrons are the electrons in the outermost shell of an atom, and they dictate the atom's chemical reactivity.
- Octet Rule: Many atoms follow the octet rule, aiming to achieve a stable configuration of eight valence electrons by gaining, losing, or sharing electrons.
- Bond Formation: Valence electrons participate in chemical bond formation, either by sharing (covalent bonds) or transferring (ionic bonds) to achieve a stable electron configuration.
- Reactivity: The number and arrangement of valence electrons influence the reactivity of an atom. Atoms with unpaired valence electrons are more reactive and readily form bonds to attain a stable configuration.
Electron Pairs
In the realm of Lewis dot structure tutorials, the concept of electron pairs, both shared and unshared, plays a pivotal role in understanding chemical bonding and molecular structure.
- Shared Pairs:
Electrons that are shared between two atoms, forming a covalent bond. Shared pairs contribute to the stability and strength of the bond.
- Unshared Pairs (Lone Pairs):
Electrons that are not involved in bonding and reside on a single atom. Lone pairs influence molecular geometry and can participate in various chemical interactions.
- Octet Rule:
Many atoms strive to achieve a stable configuration of eight valence electrons, known as the octet rule. This rule guides the formation of electron pairs and the overall stability of molecules.
- Resonance Structures:
In certain molecules, multiple valid Lewis structures can be drawn, each with different arrangements of electron pairs. These resonance structures contribute to the overall stability and properties of the molecule.
The concept of electron pairs underpins the understanding of Lewis dot structures and provides insights into molecular bonding, geometry, and reactivity. Lone pairs, in particular, can influence molecular polarity and participate in various interactions, such as hydrogen bonding and coordination bonding. Furthermore, the distribution of electron pairs around an atom determines its hybridization, which in turn affects molecular shape and bonding properties.
Octet Rule
Within the realm of Lewis dot structure tutorials, the octet rule stands as a fundamental principle that guides the arrangement of electrons in molecules and influences their overall stability. This rule states that atoms tend to gain, lose, or share electrons until they achieve a stable configuration of eight valence electrons, resembling the electron configuration of noble gases.
- Electron Configuration:
The octet rule reflects the tendency of atoms to achieve a stable electron configuration, with eight valence electrons arranged in the outermost shell.
- Stability:
Atoms with a complete octet of valence electrons exhibit increased stability and lower chemical reactivity due to the strong electrostatic attraction between the nucleus and the valence electrons.
- Bond Formation:
The octet rule drives the formation of chemical bonds as atoms strive to attain a stable octet. This can occur through the sharing or transfer of electrons, leading to the formation of covalent and ionic bonds, respectively.
- Exceptions:
While the octet rule is widely applicable, there are exceptions, particularly among elements in the first and second periods of the periodic table. For instance, hydrogen typically achieves a stable configuration with two valence electrons, while beryllium and boron form stable compounds with less than eight valence electrons.
The octet rule serves as a cornerstone in understanding the behavior of atoms and molecules. It provides a framework for predicting molecular geometry, polarity, and reactivity, enabling chemists to rationalize and interpret a wide range of chemical phenomena. Furthermore, the octet rule finds applications in various fields, including inorganic chemistry, organic chemistry, and biochemistry, contributing to the development of new materials, drugs, and technologies.
Exceptions
Within the framework of Lewis dot structure tutorials, an intriguing aspect arises: not all atoms adhere to the octet rule. This section delves into the exceptions to this prevalent principle, exploring the reasons behind these deviations and their implications for understanding molecular behavior.
- Incomplete Octet:
Certain atoms, especially in the first and second periods of the periodic table, exhibit stable Lewis structures with less than eight valence electrons. Boron and beryllium are notable examples, forming stable compounds with only six valence electrons.
- Expanded Octet:
In contrast, some heavier atoms can accommodate more than eight valence electrons, exceeding the octet rule. This is observed in elements like phosphorus and sulfur, which can form stable compounds with ten or twelve valence electrons, respectively.
- Steric Effects:
In certain molecules, steric hindrance, or the repulsion between bulky atoms or groups, can prevent the attainment of an octet. This can lead to the formation of Lewis structures with less than eight valence electrons, as seen in overcrowded molecules like neopentane.
- Resonance Structures:
Resonance structures, which represent multiple valid Lewis structures for a molecule, can contribute to deviations from the octet rule. By delocalizing electrons, resonance structures can distribute the electron density over several atoms, resulting in non-integer formal charges and non-octet configurations.
These exceptions to the octet rule highlight the dynamic nature of chemical bonding and the intricacies of molecular structure. Understanding these deviations is crucial for accurately predicting molecular properties, reactivity, and behavior. Furthermore, these exceptions underscore the importance of considering additional factors, such as steric effects and resonance, when analyzing Lewis dot structures.
Resonance
Within the realm of Lewis dot structure tutorials, the concept of resonance emerges as a pivotal aspect, offering a deeper understanding of molecular behavior and electronic configurations.
Cause and Effect: Resonance plays a crucial role in Lewis dot structure tutorials, enabling the depiction of multiple valid Lewis structures for certain molecules. This phenomenon arises when a single Lewis structure fails to adequately represent the delocalization of electrons within a molecule. By considering resonance, chemists can more accurately portray the electronic structure and properties of molecules.
Components: Resonance is an essential component of Lewis dot structure tutorials, providing a framework for understanding the distribution of electrons in molecules. It complements the fundamental principles of Lewis dot structures by allowing for the representation of multiple contributing structures that collectively describe the overall electronic configuration.
Examples: Benzene, a ubiquitous aromatic hydrocarbon, serves as a compelling example of resonance. The six carbon atoms in benzene form a ring with alternating single and double bonds. However, no single Lewis structure can accurately represent the delocalized electrons in the benzene ring. Instead, resonance structures are employed to depict the equal distribution of electrons among the carbon atoms, providing a more accurate representation of the molecule's electronic structure.
Applications: Understanding resonance is of great significance in various applications. In organic chemistry, it aids in explaining the stability, reactivity, and properties of organic molecules. Resonance also plays a crucial role in inorganic chemistry, particularly in the study of coordination complexes and metal-ligand interactions. Furthermore, the concept of resonance finds applications in materials science, biochemistry, and pharmaceutical chemistry.
Conclusion: Resonance, as a fundamental aspect of Lewis dot structure tutorials, offers a deeper comprehension of molecular electronic structures and properties. It enables chemists to depict the delocalization of electrons, providing more accurate representations of molecular behavior. Despite the complexity associated with resonance structures, their importance in understanding and predicting chemical phenomena cannot be overstated.
Formal Charge
Within the realm of Lewis dot structure tutorials, the concept of formal charge emerges as a crucial aspect, offering insights into the electronic distribution and stability of molecules.
Cause and Effect:Formal charge plays a pivotal role in Lewis dot structure tutorials by enabling the assignment of formal charges to atoms within a Lewis structure. This assignment directly influences the understanding of molecular properties and behavior. By calculating formal charges, chemists can evaluate the stability of Lewis structures, predict the polarity of molecules, and identify potential reaction sites.
Components:Formal charge is an essential component of Lewis dot structure tutorials, as it provides a quantitative measure of the electron distribution within a molecule. It is calculated by considering the difference between the number of valence electrons an atom possesses in its neutral state and the number of electrons assigned to it in the Lewis structure. Understanding formal charge allows chemists to assess the electronic stability of molecules and identify atoms with significant charge imbalances.
Examples:Consider the Lewis structure of water (H2O) as an example. By assigning formal charges to each atom, we find that oxygen has a formal charge of -1, while each hydrogen atom has a formal charge of +1. This indicates that oxygen has a partial negative charge, while the hydrogen atoms have partial positive charges. This charge distribution helps explain the polarity of the water molecule and its ability to participate in hydrogen bonding.
Applications:Understanding formal charge has significant practical implications in various fields. In organic chemistry, it aids in determining the reactivity and stability of organic compounds. In inorganic chemistry, it helps predict the bonding and coordination behavior of metal complexes. Furthermore, formal charge calculations are essential in computational chemistry and drug design, where accurate representations of molecular electronic structures are crucial.
Summary:Formal charge, as a fundamental aspect of Lewis dot structure tutorials, provides valuable insights into the electronic distribution and stability of molecules. By assigning formal charges to atoms, chemists can evaluate molecular properties, predict polarity, and identify reaction sites. Despite the challenges in accurately assigning formal charges in complex molecules, its significance in understanding chemical behavior cannot be overstated. Formal charge remains an indispensable tool for chemists seeking to unravel the intricacies of molecular interactions and design new materials with tailored properties.
Challenges
Lewis dot structure tutorials provide a valuable foundation for understanding molecular structure and bonding. However, the realm of chemistry encompasses complex molecules that pose significant challenges in accurately depicting their electronic structures. This section explores the intricate relationship between the challenges presented by complex molecules and the techniques employed in Lewis dot structure tutorials to address these complexities.
Cause and Effect: The intricate nature of complex molecules necessitates the utilization of advanced techniques in Lewis dot structure tutorials. These techniques enable chemists to overcome the limitations of basic Lewis dot structures and obtain more accurate representations of molecular electronic structures. By delving into these advanced techniques, tutorials empower learners to tackle the complexities of real-world molecules.
Components: Advanced techniques serve as essential components of Lewis dot structure tutorials, extending the capabilities of basic tutorials and enabling the exploration of complex molecular systems. These techniques include resonance structures, formal charges, and molecular orbital theory, among others. Each technique plays a crucial role in providing a comprehensive understanding of molecular structure and bonding, thus enhancing the overall effectiveness of Lewis dot structure tutorials.
Examples: The application of advanced techniques in Lewis dot structure tutorials manifests in various real-life instances. In organic chemistry, resonance structures are employed to depict the delocalization of electrons in molecules like benzene, providing insights into their stability and reactivity. Formal charges are utilized to determine the polarity of molecules, guiding the prediction of intermolecular interactions and molecular properties. Additionally, molecular orbital theory offers a sophisticated framework for understanding the electronic structure and bonding in complex inorganic and organometallic compounds.
Applications: Understanding the challenges posed by complex molecules and the techniques used to address them in Lewis dot structure tutorials holds immense practical significance. These advanced techniques empower chemists to design and synthesize novel materials with tailored properties, unlocking new possibilities in fields such as medicine, energy storage, and electronics. Furthermore, they contribute to the development of accurate computational models that aid in drug discovery, catalysis, and materials science.
In conclusion, the challenges presented by complex molecules drive the need for advanced techniques in Lewis dot structure tutorials. These techniques unveil the intricacies of molecular structure and bonding, facilitating the understanding and prediction of chemical properties and reactivity. The interplay between challenges and techniques underscores the dynamic nature of chemistry and highlights the importance of continuous learning and adaptation in this ever-evolving field.
Frequently Asked Questions (FAQs)
This section addresses common questions and clarifies aspects related to the topic of Lewis dot structure tutorials:Question 1: What is the significance of Lewis dot structure tutorials?
Answer: Lewis dot structure tutorials provide a foundational understanding of molecular structure and bonding, enabling the visualization and analysis of electron arrangements in molecules. They serve as a cornerstone for comprehending chemical properties, predicting reactivity, and designing new materials.
Question 2: Are Lewis dot structures limited to simple molecules?
Answer: While Lewis dot structures are commonly used for simple molecules, advanced techniques can be employed to represent complex molecules. Resonance structures, formal charges, and molecular orbital theory extend the capabilities of Lewis dot structures to accurately depict the electronic configurations of diverse molecular systems.
Question 3: How do Lewis dot structures contribute to understanding chemical reactions?
Answer: Lewis dot structures provide insights into the electronic changes that occur during chemical reactions. By analyzing the electron arrangements of reactants and products, chemists can predict reaction mechanisms, identify reactive sites, and determine the stability of reaction intermediates.
Question 4: What are the limitations of Lewis dot structures?
Answer: Lewis dot structures primarily represent valence electrons and do not explicitly account for molecular orbitals or electron delocalization. In certain cases, resonance structures or molecular orbital theory may be necessary for a more accurate depiction of molecular electronic structures.
Question 5: How can I improve my skills in drawing Lewis dot structures?
Answer: Practice and understanding the underlying principles are key. Start with simple molecules and gradually progress to more complex structures. Refer to periodic trends, electronegativity values, and resonance concepts to enhance your accuracy and proficiency.
Question 6: What are the applications of Lewis dot structures beyond academia?
Answer: Lewis dot structures find practical applications in various fields, including drug design, materials science, and catalysis. They aid in understanding intermolecular interactions, predicting molecular properties, and designing new compounds with tailored functionalities.
These FAQs provide a concise overview of the key aspects and applications of Lewis dot structure tutorials. Understanding these concepts lays the groundwork for further exploration of molecular structure, bonding, and reactivity in the realm of chemistry.TIPS
This section provides a comprehensive guide to mastering the effective representation of Lewis dot structures, enabling clear communication and a deeper understanding of molecular structures and bonding.
Tip 1: Grasp the Fundamentals:
Begin by understanding the basics of Lewis dot structures, including the representation of valence electrons, the octet rule, and the concept of formal charges. A solid foundation will facilitate the accurate depiction of molecular electronic configurations.
Tip 2: Utilize Periodicity Trends:
Leverage the periodic trends of elements to predict the number of valence electrons and the tendency to gain or lose electrons. This knowledge aids in determining the Lewis dot symbols for various atoms.
Tip 3: Apply the Octet Rule Wisely:
Remember that the octet rule, while generally applicable, may have exceptions. Some elements, like hydrogen and boron, exhibit stable configurations with less than eight valence electrons.
Tip 4: Handle Multiple Bonds with Care:
When representing multiple bonds, ensure that you account for the appropriate number of shared electron pairs. Double and triple bonds involve two and three pairs, respectively.
Tip 5: Address Formal Charges Accurately:
Assign formal charges to atoms in a Lewis structure to assess their electronic stability. Formal charges help identify potential reaction sites and polarity within molecules.
Tip 6: Employ Resonance Structures Strategically:
In cases where a single Lewis structure fails to adequately represent a molecule's electronic configuration, utilize resonance structures. These alternative structures provide a more accurate depiction of electron delocalization.
Tip 7: Practice Regularly:
Regular practice is essential for mastering Lewis dot structure representation. Engage in exercises that involve drawing structures for various molecules, reinforcing your understanding and identifying patterns.
Summary:
By following these tips, you can effectively represent Lewis dot structures, gaining valuable insights into molecular structures, bonding, and reactivity. This understanding forms the foundation for further exploration and application of chemical concepts.
Transition to Conclusion:
As you delve deeper into the world of Lewis dot structures, you'll discover their significance in predicting chemical properties, understanding reaction mechanisms, and designing new materials. These versatile representations serve as a cornerstone for unraveling the intricate world of molecular interactions and driving advancements across scientific disciplines.
Conclusion
Our exploration of "Lewis Dot Structure Tutorial" illuminated the significance of these diagrams in comprehending molecular structures, bonding patterns, and chemical behavior. Key insights emerged throughout the article, highlighting the interconnectedness of fundamental concepts.
Firstly, Lewis dot structures provide a visual representation of valence electrons, enabling chemists to predict molecular properties, reactivity, and bonding arrangements. This understanding underpins various fields, including drug design, materials science, and catalysis.
Secondly, the concept of resonance structures proved crucial in depicting the delocalization of electrons in certain molecules. By considering multiple valid Lewis structures, chemists can better explain molecular stability and properties, unlocking insights into complex chemical phenomena.
Finally, the assignment of formal charges aided in evaluating the electronic stability of molecules and identifying reaction sites. This knowledge empowers chemists to predict reaction pathways, design new materials, and optimize chemical processes.
As we conclude our journey into Lewis dot structure tutorials, let us remember the profound impact these representations have on our understanding of the molecular world. They serve as a gateway to unraveling the intricacies of chemical bonding, guiding scientific advancements and shaping our technological landscape. The exploration of Lewis dot structures is an ongoing pursuit, beckoning us to delve deeper into the fascinating realm of molecular interactions.